
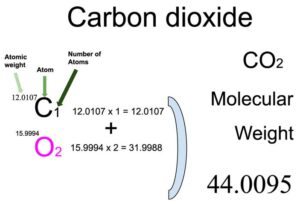
The acid dissociation constant for carbonic acid K a = 4.45 x 10 -7 mol dm -3. Carbon dioxide reacts with water in a reversible reaction to form carbonic acid (H 2CO 3).
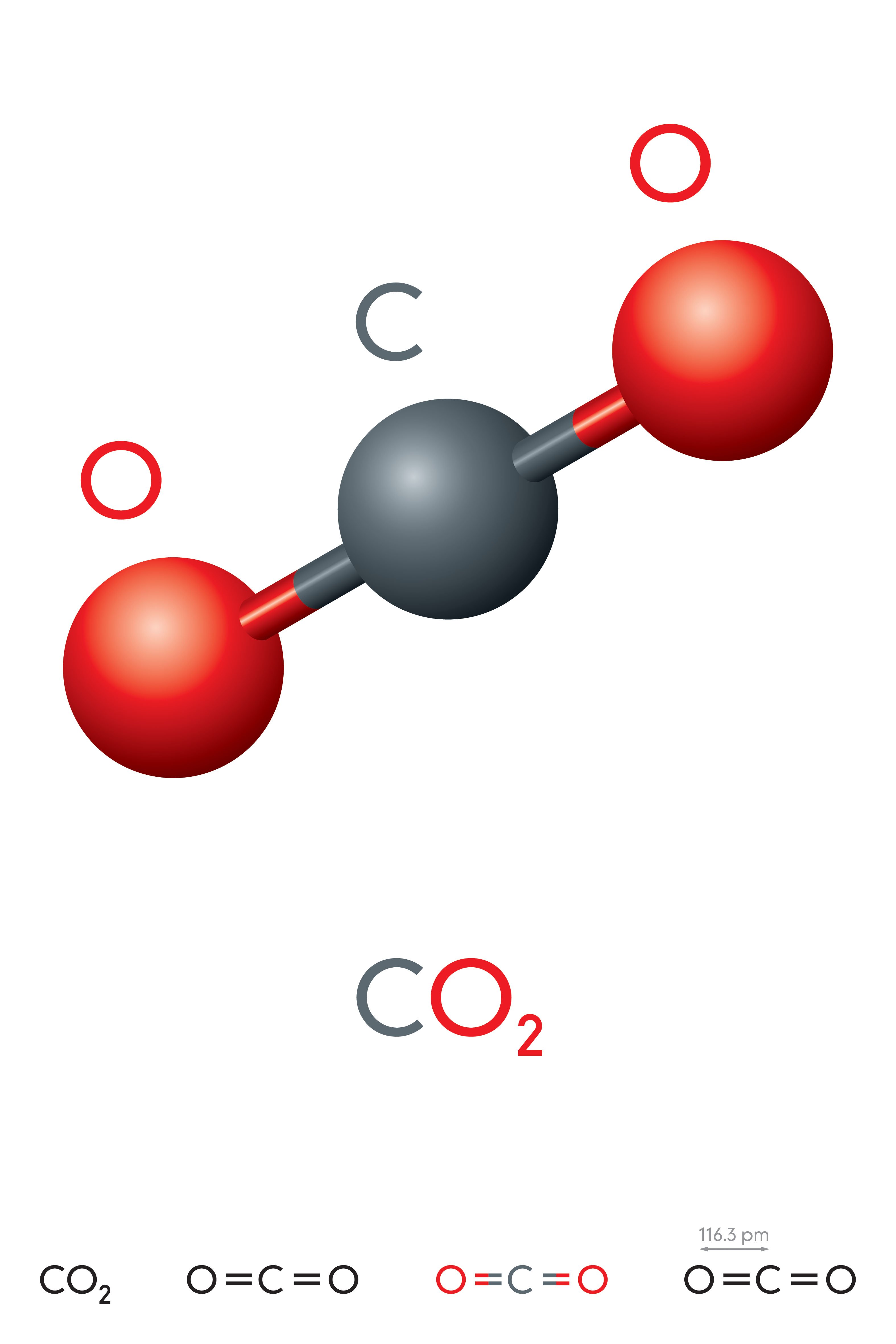
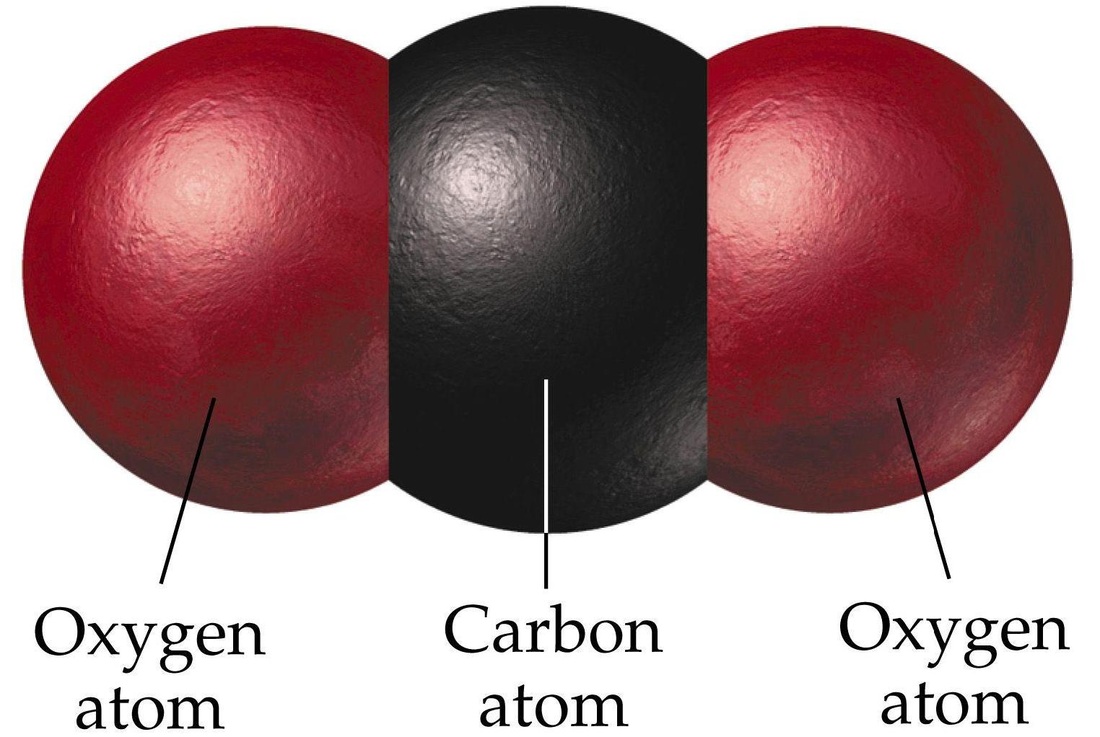
The size of the 'electron drift' is indicated by the size of the blue arrows.ĭiamond is an allotrope of carbon which was first confirmed in a very expensive experiment performed by the English chemist Smithson Tennant who was the first to burn a diamond in air (~600-800☌) producing carbon dioxide.Ĭarbon dioxide as a non-metallic oxide is acidic. Magnesium's smaller 2+ ion (72 pm) polarises the carbonate 2- ion (135 pm) more than the larger barium 2+ ion (100 pm) because it has greater attractions for its electrons. These differences can be explained in terms of charge-density differences for the cations (M 2+). The trend is that on descending Group 2 their carbonates become more difficult to decompose thermally as reflected by the increasing temperatures. Interestingly, beryllium carbonate is unstable under standard conditions (298 K, 100 kPa) and the subsequent Group 1 carbonates are thermally stable. The thermal decomposition of lithium carbonate and the following Group 2 carbonates leads to the production of carbon dioxide gas and the metal oxide. This simple chemistry has been exploited in craft such as submarines and space shuttles where the build up of carbon dioxide from respiration needs tackling.ĬO 2 (g) + 2 NaOH (aq) Na 2CO 3 (aq) + H 2O (l) If you see a white crust around the stoppers inform the technicians who need to supply fresh solutions. Next time you are in the laboratory check the top of the sodium hydroxide bottles. ReactionsĬarbon dioxide is an acidic oxide (a typical property of the majority of non-metal oxides) and reacts with sodium hydroxide to form a salt and water. This is why dry ice should not be handled as it causes burns by freezing the skin. There are some superb films of the reaction online using blocks of dry ice (solid carbon dioxide). In the unlikely event that you are faced with a magnesium fire it is not advisable to use a carbon dioxide fire extinguisher because it actually reacts with magnesium.Ĭarbon dioxide will burn on reaction with magnesium and the result is the formation of magnesium oxide and carbon as soot. They are suitable for electrical fires in offices containing computers, televisions and photocopiers. These extinguishers have the advantage of not leaving a residue after they have been used (discharged). Carbon dioxide extinguishers are suitable for class B fires (flammable liquids and gases). This type of fire extinguisher has the added advantage of also cooling the fuel. Once this part of the fire triangle has been removed the fire is extinguished. They work by displacing the air (oxygen) from the area surrounding the fire. Carbon dioxide is non-polar as its dipoles cancel, and this contrasts with the polar molecule sulfur dioxide (SO 2).Ĭarbon dioxide fire extinguishers contain highly pressurized carbon dioxide which is a non-flammable inert gas, which acts as a smothering material. This is why it has a very low melting point 217 K (-56☌) at 5.2 atmospheres. Solid carbon dioxide has weak forces (van der Waals) between molecules which holds them together. It is an unusual solid as it sublimes (turning from solid to gas without going through the liquid state). Solid carbon dioxide is known by chemists as cardice and by everyone else as dry ice. This gas was 'discovered' by Scottish scientist (physicist and chemist) Joseph Black (right). Carbon dioxide is a linear molecule with a bond angle of 180°. At room temperature carbon dioxide is a colourless gas which has a slightly sweet smell. Carbon dioxide has the formula CO 2 and at the centre of this linear molecule is a carbon atom joined by two pairs of double-bonds to the oxygen atoms, i.e O=C=O. Carbon Dioxide - Molecule of the Month - May 2012 - HTML-only versionĬarbon Dioxide The gas we exhale that's both a Greenhouse gasĬarbon dioxide is a simple covalent molecule that most people have heard about, as it is often in the news due to its role in global warming.


 0 kommentar(er)
0 kommentar(er)
